Introduction: Our phase I graft-versus-host disease (GVHD) prevention trial of pacritinib (recommended phase II dose: 100mg po BID day 0 to +70, dose level 2) plus sirolimus (8-14ng/ml) and tacrolimus (3-7ng/ml) (PAC/SIR/TAC) demonstrated the regimen was safe and free of pan-JAK myelosuppression after allogeneic hematopoietic cell transplantation (alloHCT). PAC inhibits JAK2 with no activity against JAK1, avoiding off-target suppression of IL-2 required by Tregs. JAK2/STAT3 activity mediates IL-6, IL-12, and IL-23 receptor signaling and subsequent pathogenic Th1 and Th17 differentiation. JAK2/mTOR blockade supports Treg potency, providing further rationale for the PAC/SIR/TAC combination. Herein we report on our completed phase II trial of PAC/SIR/TAC after 8/8-HLA matched alloHCT.
Methods: This single-arm phase II trial (NCT02891603) was powered to determine if PAC/SIR/TAC suppressed %pSTAT3 + CD4 + T cells at day +21 (primary endpoint: %pSTAT3 + CD4 + T cells ≤ 35%) and determine the cumulative incidence of grade II-IV acute GVHD by day +100. We also evaluated the impact of PAC/SIR/TAC on CD4 T cell differentiation (Treg, Th1, Th17) and related CD28 (pS6 and pH3ser10) and IL-2 receptor (pSTAT5) signal transduction after alloHCT. Eligible patients (n=28) received alloHCT for AML, MDS, ALL, and MF. Reduced (n=21) or myeloablative (n=7) intensity conditioning was permitted. HLA-A, -B, -C, and -DRB1 matched-related (n=7) or unrelated donors (n=21) were allowed. Adequate vital organ function and Karnofsky performance status (KPS ≥ 80%) were required.
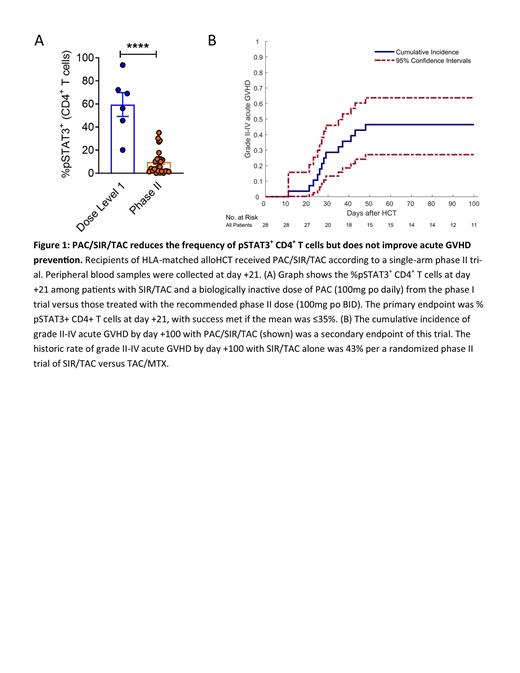
Results: PAC/SIR/TAC (PAC 100mg BID) met the primary endpoint of the phase II study, reducing %pSTAT3 + CD4 + T cells to 9.62% at day +21 ( Figure 1A). PAC/SIR/TAC significantly improved CD4 + T cell STAT5 phosphorylation at day +21, increasing the ratio of pSTAT5 + to pSTAT3 + CD4 + T cells (ratio 80.7 v 1.71 P<0.0001) compared to dose level 1 PAC/SIR/TAC of the phase I trial that lacked effective JAK2 blockade (PAC 100mg daily, day +21 %pSTAT3 + CD4 + T cells 59.5%). Partial CD28 signaling blockade was achieved by PAC/SIR/TAC compared to dose level 1, with suppression of mTOR (%pS6 + CD4 + T cells 7.67 v 22.5% P=0.0009) but only modest inhibition of Aurora kinase A activity (%pH3ser10 + CD4 + T cells 71.3 v 98.6% P<0.0001), a pathway for escape alloreactivity. Th1 (0.01 v 0.03 k/µl P=0.026) and Th17 (0.016 v 0.032 P=0.031) cells were reduced at day +21, increasing the ratio of Tregs to Th1 and Th17 cells (0.84 v 0.21 P=0.043) with PAC/SIR/TAC compared to dose level 1. Like JAK2 KO murine T cells, Th2 cells at day +21 were increased with PAC/SIR/TAC (0.056 v 0.001 k/µl P=0.036), compared to dose level 1. T, B, and NK cell engraftment at day +21 was comparable to dose level 1. Despite suppression of Th1 and Th17 cells, the cumulative incidence of grade II-IV acute GVHD by day +100 with PAC/SIR/TAC was similar to historic SIR/TAC values (46.4 v 43%) ( Figure 1B). Acute GVHD onset did not correlate with duration of PAC therapy, depth of pSTAT3 inhibition, or burden of circulating Th1, Th17, or Th2 cells.
Conclusions: While PAC/SIR/TAC successfully reduced pSTAT3, increased pSTAT5, and suppressed Th1 and Th17 cells, the regimen did not reduce acute GVHD risk. Completed phase II and III trials testing tocilizumab (anti-IL-6 monoclonal antibody, ACTRN12612000726853, ACTRN12614000266662) and now PAC reveal a biologic disconnect between effective IL-6/JAK2/pSTAT3 axis blockade and a disappointing lack of clinical improvement in acute GVHD prevention. We surmise uncontrolled T cell Aurora kinase A activity contributed to acute GVHD via CD28 costimulation in this trial. As PAC polarizes the pSTAT5:pSTAT3 CD4 + T cell ratio and targets IRAK1 and CSFR1, it may have activity in refractory chronic GVHD. This concept is now being tested by others (NCT05531786).
OffLabel Disclosure:
Pidala:Moffitt Cancer Center: Other: Personal fees from Syndax, Amgen, and Regeneron outside the submitted work. Holtan:Vitrac: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Sanofi: Research Funding; CSL Behring: Other: Endpoint Adjudication Committee. Elmariah:Bristol Myers Squibb: Research Funding. Bejanyan:CTI BioPharma: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; CRISPR: Consultancy, Research Funding; CareDx Pharma: Consultancy, Research Funding; Orca Bio: Consultancy, Research Funding; Medexus Pharmaceuticals: Consultancy, Research Funding; Magenta: Consultancy, Research Funding. Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Faramand:Kite: Research Funding; Gilead: Research Funding. Davila:Capstan: Other: Advisor or review panel participant; Caribou Biosciences: Consultancy; Kite Pharma Inc.: Other: Teaching and Speaking; Legend Biotech: Consultancy; Precision Biosciences: Other: Ownership interest (stock, stock options in a publicly owned company); Syncopation Life Sciences: Consultancy; Synthekine: Consultancy; Bellicum Pharmaceuticals, Inc.: Other: Advisor or review panel participant; Ownership interest (stock, stock options in a publicly owned company); CRISPR (CRSP): Patents & Royalties: Intellectual property rights (Royalties or patent sales); Atara Biotherapeutics: Consultancy; Adaptive Biotechnologies: Other: Ownership interest (stock, stock options in a publicly owned company); Adicet: Consultancy. Blazar:BlueRock Therapeutics: Current Employment, Membership on an entity's Board of Directors or advisory committees; Carisma Therapeutics: Current Employment, Research Funding. Miller:Vycellix: Consultancy, Current holder of stock options in a privately-held company; Sanofi: Membership on an entity's Board of Directors or advisory committees; GT BioPharma: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding; Fate Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding. Bachanova:Citius: Research Funding; BMS: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB; Incyte: Research Funding; Gamida Cell: Research Funding. Betts:CRISPR Therapeutics: Patents & Royalties; Incyte: Consultancy; CTI BioPharma: Consultancy; Vitrac: Research Funding.
Pacritinib is a JAK2 inhibitor FDA-approved for the treatment of adults with intermediate or high-risk primary or secondary (post-polycythemia vera [PPV] or post-essential thrombocythemia [PET]) myelofibrosis (MF) with a platelet count below 50 Ã- 109/L. Off-label use of pacritinib will be discussed in the context of GVHD prevention.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal